SIMPLICEF™ TABLETS
Pharmacia & Upjohn
(cefpodoxime proxetil)
Antimicrobial For Oral Use In Dogs Only
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
DESCRIPTION
Cefpodoxime proxetil is an orally administered, extended spectrum, semi-synthetic cephalosporin antibiotic. The chemical name is: (+/-)-1-Hydroxyethyl(+)-(6R,7R)-7-[2-
(2-amino-4-thiazolyl)glyoxylamido]-3-methoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0] oct-2-ene-2-carboxylate, 72-(Z)-(0-methyloxime), isopropyl carbonate (ester) [87239-81-4].
Cefpodoxime proxetil Chemical Structure:
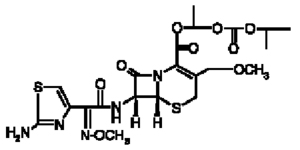
Cefpodoxime proxetil is a prodrug; its active metabolite is cefpodoxime. All doses of SIMPLICEF (cefpodoxime proxetil) tablets are expressed in terms of the active cefpodoxime moiety. Simplicef is available as:
100 mg Tablet, each reddish-orange, ellipital, scored tablet contains cefpodoxime proxetil equivalent of 100 mg of cefpodoxime.
200 mg Tablet, each light orange, elliptical tablet contains cefpodoxime proxetil equivalant to 200 mg of cefpodoxime.
INDICATION: SIMPLICEF tablets are indicated for the treatment of skin infections (wounds and abscesses) in dogs caused by susceptible strains of Staphylococcus intermedius, Staphylococcus aureus, Streptococcus canis (group G, B hemolytic), Escherichia coli, Pasteurella multocida, and Proteus mirabilis.
DOSAGE AND ADMINISTRATION:
Dose range: The dose range of SIMPLICEF (cefpodoxime proxetil) tablets is 5-10 mg/kg (2.3-4.5 mg/lb) body weight, administered orally, once a day. The dose may be given with or without food. The determination of dosage for any particular patient must take into consideration such factors as the severity and nature of the infection, the susceptibility of the causative organisms, and the integrity of the patient's host-defense mechanisms. Obtain a sample of the pathogenic organism for culture and sensitivity testing prior to beginning antimicrobial therapy. Once results become available, continue with appropriate therapy.
Duration: SIMPLICEF tablets should be administered once daily for 5-7 days or for 2-3 days beyond the cessation of clinical signs, up to a maximum of 28 days. Treatment of acute infections should not be continued for more than 3-4 days if no response to therapy is seen.
Dosing Charts: For daily oral administration of SIMPLICEF at 5 mg/kg (Table 1) and 10 mg/kg (Table 2).
Table 1. Dose Table for SIMPLICEF Tablets at 5 mg/kg Total Daily Dosage
|
Weight of Dog (lbs)
|
|
Daily Dose
|
22
|
44
|
66
|
88
|
132
|
|
No. of 100 mg tablets
|
0.5
|
1
|
1.5
|
|
1
|
|
No. of 200 mg tablets
|
|
|
|
1
|
1
|
|
Weight of Dog (kgs)
|
|
Daily Dose
|
10
|
20
|
30
|
40
|
60
|
|
No. of 100 mg tablets
|
0.5
|
1
|
1.5
|
|
1
|
|
No. of 200 mg tablets
|
|
|
|
1
|
1
|
Table 2. Dose Table for SIMPLICEF Tablets at 10 mg/kg Total Daily Dosage
|
Weight of Dog (lbs)
|
|
Daily Dose
|
11
|
22
|
44
|
66
|
88
|
132
|
|
No. of 100 mg tablets
|
0.5
|
1
|
|
1
|
|
|
|
No. of 200 mg tablets
|
|
|
1
|
1
|
2
|
3
|
|
Weight of Dog (kgs)
|
|
Daily Dose
|
5
|
10
|
20
|
30
|
40
|
60
|
|
No. of 100 mg tablets
|
0.5
|
1
|
|
1
|
|
|
|
No. of 200 mg tablets
|
|
|
1
|
1
|
2
|
3
|
CONTRAINDICATIONS
Cefpodoxime proxetil is contraindicated in dogs with known allergy to cefpodoxime or to the �-lactam (penicillins and cephalosporins) group of antibiotics.
WARNINGS
Not for human use. Keep this and all drugs out of reach of children. Antimicrobial drugs, including penicillins and cephalosporins, can cause allergic reactions in sensitized individuals. To minimize the possibility of allergic reactions, those handling such antimicrobials, including cefpodoxime, are advised to avoid direct contact of the product with the skin and mucous membranes.
PRECAUTIONS
The safety of cefpodoxime proxetil in dogs used for breeding, pregnant dogs, or lactating bitches has not been demonstrated. As with other cephalosporins, cefpodoxime proxetil may occasionally induce a positive direct Coombs' test.
ADVERSE REACTIONS
A total of 216 dogs of various breeds and ages ranging from 2 months to 15 years were included in the field study safety analysis. The following table shows the number of dogs displaying each clinical observation.
Table 3. Abnormal Health Findings in the U.S. Field Study1
|
Clinical
Observation
|
SIMPLICEF
(n=118)
|
Active Control
(n=98)
|
|
Vomiting
|
2
|
4
|
|
Diarrhea
|
1
|
1
|
|
Increased water drinking
|
0
|
2
|
|
Decreased appetite
|
1
|
1
|
1 Dogs may have experienced more than one of the observations during the study.
To report a suspected adverse reaction call 1-800-366-5288.
To request a material safety data sheet (MSDS) for SIMPLICEF tablets, call 1-800-733-5500.
CLINICAL PHARMACOLOGY
Pharmacokinetics/Pharmacodynamics: Cefpodoxime proxetil is a prodrug that is absorbed from and de-esterified in the gastrointestinal tract to its active metabolite, cefpodoxime. Following oral administration to fasting Beagles, oral bioavailability was 63.1 � 5.3%.
Figure 1. Canine Plasma Concentration of Cefpodoxime After a Single Oral Dose of 10 mg/kg Cefpodoxime Proxetil Tablets
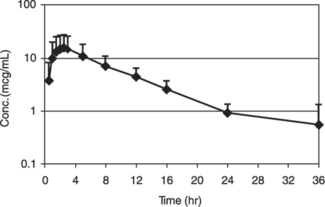
Cefpodoxime is distributed in the body with an apparent volume of distribution of 151 � 27 mL/kg. Like other �-lactam antibiotics, cefpodoxime is eliminated from the body primarily in the urine, with an apparent elimination half-life of approximately 5-6 hours after oral administration. This is similar to the 4.7 hour apparent elimination half-life observed after intravenous dosing. Following intravenous administration of 10 mg/kg, the average total body clearance (ClB) was 22.7 � 4.19 mL/hr/kg.
Table 4. Summary of Pharmacokinetic Parameters Obtained after a Single Oral Dose of 10 mg Cefpodoxime/kg BW, Administered as a Tablet
|
PK Parameter
|
Unit
|
Tablet ISD)
|
|
AUC0-8
|
mcg�hr/mL
|
145 (77.6)
|
|
AUC0-LOQ
|
mcg�hr/mL
|
142 (77.5)
|
|
Maximum concentration (Cmax)
|
mcg/mL
|
16.4 (11.8)
|
|
Terminal plasma elimination half-life (t1/2,z)
|
hr
|
5.61 (1.15)
|
|
Time of maximum concentration (tmax)
|
hr
|
2.21 (0.542)
|
|
Mean residence time (MRT0-8)
|
hr
|
9.21(l.97)
|
Microbiology: Like other �-lactam antibiotics, cefpodoxime exerts its inhibitory effect by interfering with bacterial cell wall synthesis. This interference is primarily due to its covelently binding to the penicillin-binding proteins (PBPs) (i.e. transpeptidase and/or carboxypeptidase), which are essential for synthesis of the bacterial cell wall. Therefore, cefpodoxime is bactericidal. Cefpodoxime is stable in the presence of many common �-lactamese enzymes. As a result, many organisms resistant to other �-lactam antibiotics (penicillins and some cephalosporins) due to the production of �-lactamases may be susceptible to cefpodoxime.
Cefpodoxime has a broad spectrum of clinically useful antibacterial activity that includes staphylococci, streptococci, and Gram-negative species (including Pasteurella, Escherichia, and Proteus). The compound is not active against most obligate anaerobes, Pseudomonas spp., or enterococci. The minimum inhibitory concentrations (MICs) for cefpodoxime against Gram-positive and Gram-negative pathogens isolated from canine skin infections (wounds and abscesses) in a 2002 U.S. field study are presented in Table 5. All MICs were determined in accordance with the National Committee for Clinical Laboratory Standards (NCCLS). Appropriate quality control (QC) ranges for in vitro susceptibility testing are presented in Table 6.
Table 5. Cefpodoxime Minimum Inhibitory Concentration Values (mcg/mL) from a 2002 Field Study Evaluating Skin Infections (wounds and abscesses) of Canines in the United States.
|
Organism*
|
# of Isolates
|
MIC50
|
MIC90
|
Range
|
|
Staphylococcus intermedius
|
118
|
0.12
|
0.50
|
0.12->32.0
|
|
Streptococcus canis (group G, � hemolytic)
|
33
|
=0.03
|
=0.03
|
0.03�
|
|
Escherichia coli
|
41
|
0.25
|
0.50
|
0.12->32.0
|
|
Pasteurella multocida
|
32
|
=0.03
|
=0.03
|
=0.03-0.12
|
|
Proteus mirabilis
|
14
|
=0.03
|
0.06
|
=0.03-0.06
|
|
Staphylococcus aureus
|
19
|
2.0
|
2.0
|
0.12-2.0
|
� No Range, all isolates yielded the same value.
* Veterinary specific interpretive criteria have not been established for the above listed canine pathogens by the NCCLS atthis time.
Table 6. Acceptable Quality Control Ranges for Cefpodoxime
|
QC ATCC strain
|
KB Disk Diffusion Method
|
Broth Micro-dilution Method
|
|
Drug concentration
|
Zone diameter
|
MIC
|
|
Escherichia coli 25922
|
10 mcg
|
23-28 mma
|
0.25-1 mcg/mLa
|
|
Staphylococcus aureus 25923
|
10 mcg
|
19-25 mma
|
|
|
Staphylococcus aureus 29213
|
|
|
1-8 mcg/mLa
|
|
Streptococcus pneumoniae 49619
|
10 mcg
|
28-34 mmb
|
0.03-0.12 mcg/mLb
|
a These ranges are for quality control strains used to monitor accuracy of minimum inhibitory concentrations (MICs) of non-fastidious organisms using cation-adjusted Mueller-Hinton agar or broth medium. The dilution range should encompass the (IC ranges of these strains in the broth micro-dilution method.
b These ranges are for quality control strains used to monitor accuracy of minimum inhibitory concentrations (MICs) of fastidious organisms. When susceptibility testing is performed for Streptococcus canis (group G, � hemolytic), Streptococcus pneumoniae ATCC 49619 should be included as a QC strain in the presence of 5% lysed sheep blood (KB disk diffusion method) or 2.5% lysed horse blood (broth micro-dilution method).
EFFECTIVENESS
The clinical effectiveness of SIMPLICEF (cefpodoxime proxetil) was established in a multi-location (23 site) field study. In this study, 216 dogs with infected wounds or abscesses were treated with either SIMPLICEF (n=118) once daily at 5 mg/kg (2.3 mg/lb) body weight or with a active control antibiotic (n=98) administered twice daily for 5-7 days. In this study, SIMPLICEF was considered noninferior to the active control (88.7% versus 88.4% respectfully) in the treatment of canine skin infections (wounds and abscesses) caused by susceptible strains of Staphylococcus intermedius, Staphylococcus aureus, Streptococcus canis (group G, R hemolytic), Escherichia coli, Pasteurella multocida, and Proteus mirabilis.
ANIMAL SAFETY
In target animal safety studies, cefpodoxime was well tolerated at exaggerated daily oral doses of 100 mg/kg/day (10 times the maximum label dose) for 13 weeks in adult dogs and for 28 days in puppies (18-23 days of age). Therefore, once daily administration of cefpodoxime oral tablets at the maximum labeled dose of 10 mg/kg for up to 28 days was shown to be safe in adult dogs and puppies.
Blood dyscrasia including neutropenias, may be seen following high doses of cephalosporins. Cephalosporin administration should be discontinued in such cases.
STORAGE INFORMATION
Store tablets at controlled room temperature 20° to 25°C (68° to 77°F). Replace cap securely after each opening.
HOW SUPPLIED
SIMPLICEF tablets are available in the following strengths (cefpodoxime equivalent), colors, and sizes:
|
100 mg (reddish-orange, elliptical, scored, debossed with 5228)
|
|
Bottles of 100
|
NDC 0009-5228-01
|
|
200 mg (light orange, elliptical, debossed with 5229)
|
|
Bottles of 100
|
NDC 0009-5229-01
|
NADA # 141-232, Approved by FDA
Distributed by: Pharmacia & Upjohn Company, Division of Pfizer Inc, New York, NY 10017
Made in Belgium
Revised July 2004
819 826 001
4725-47
5R7341
NAC No.: 10490620